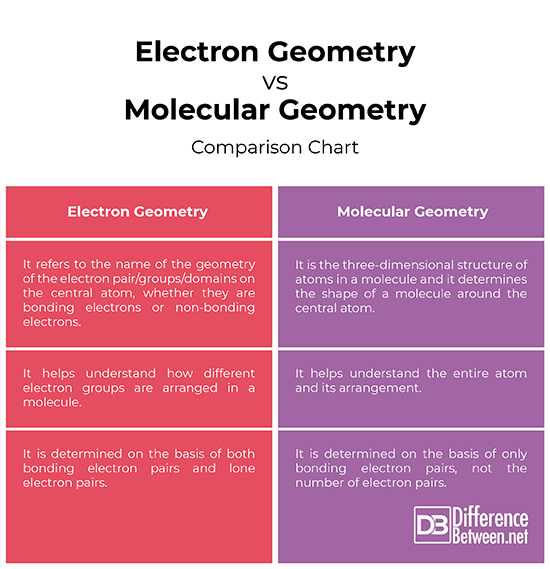
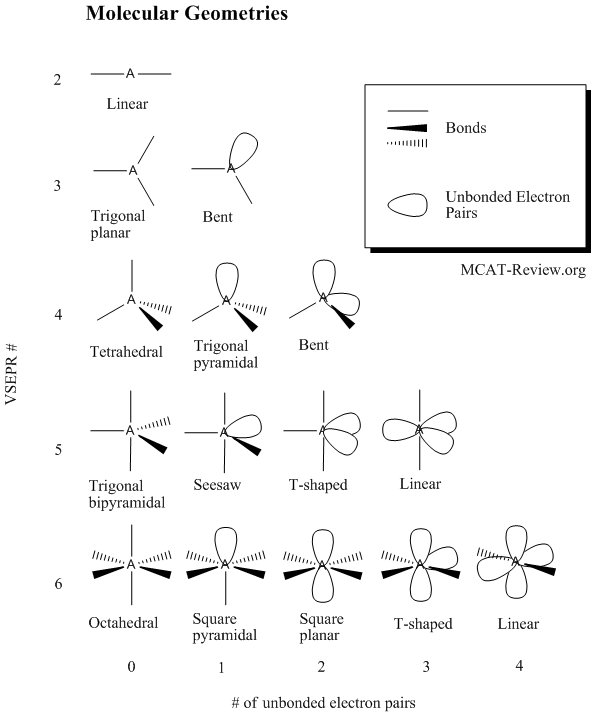
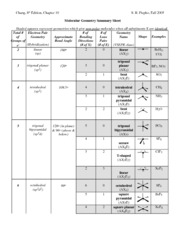
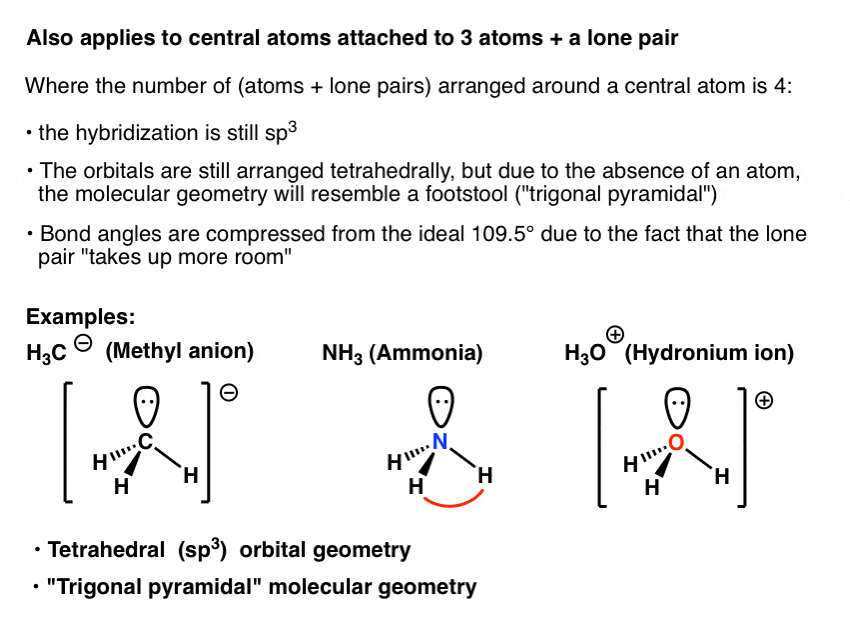
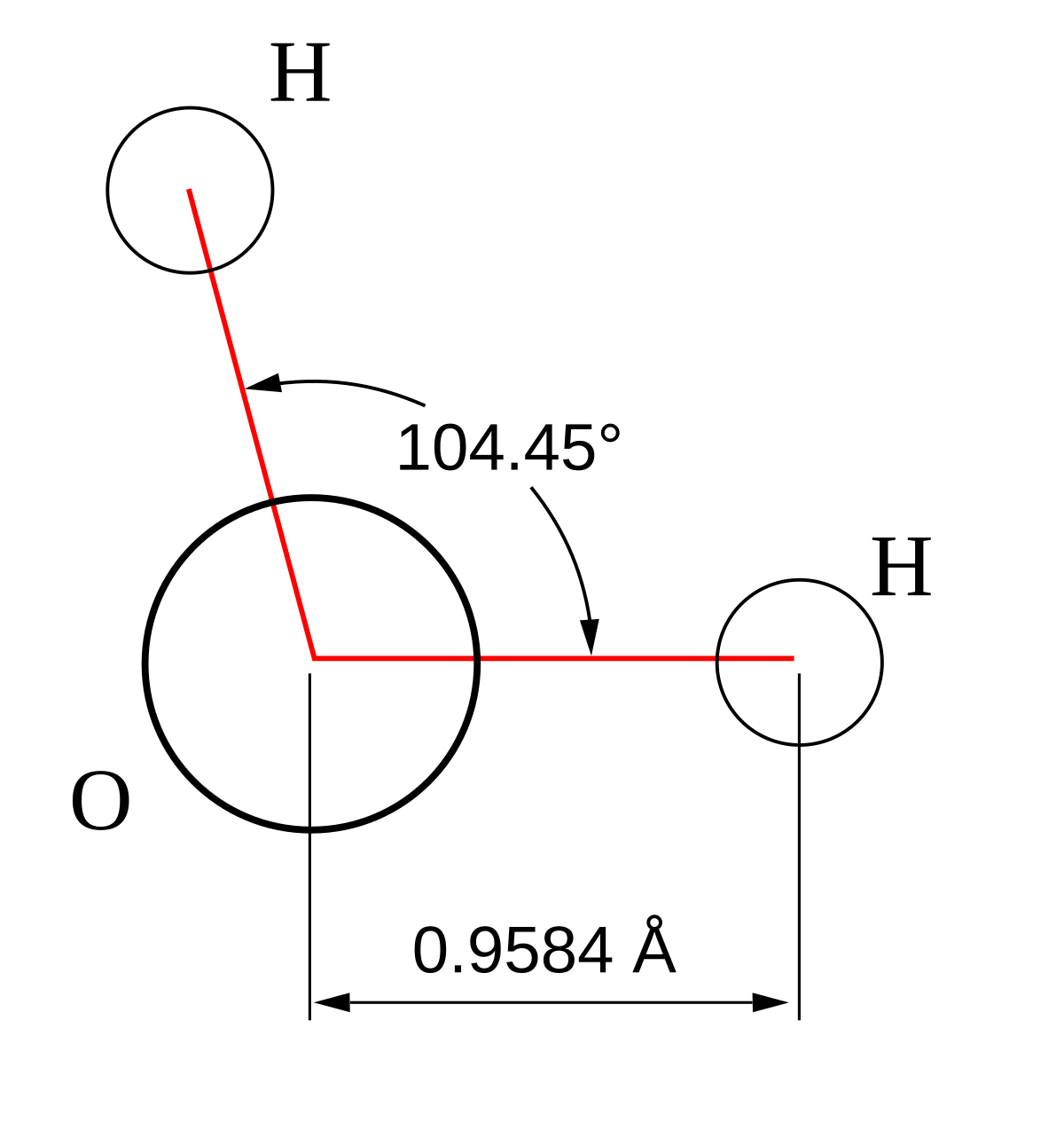
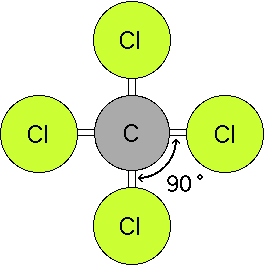
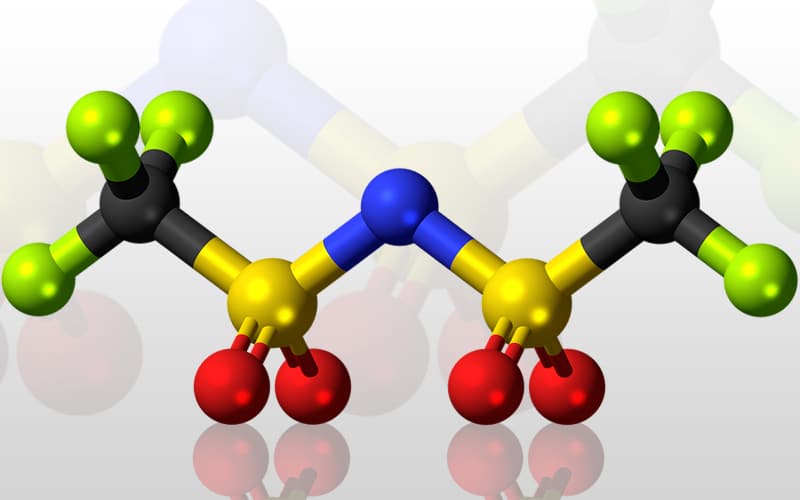
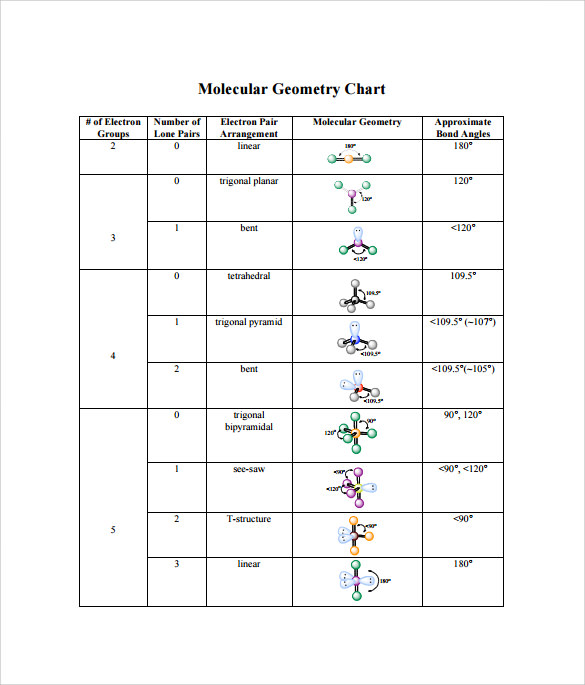
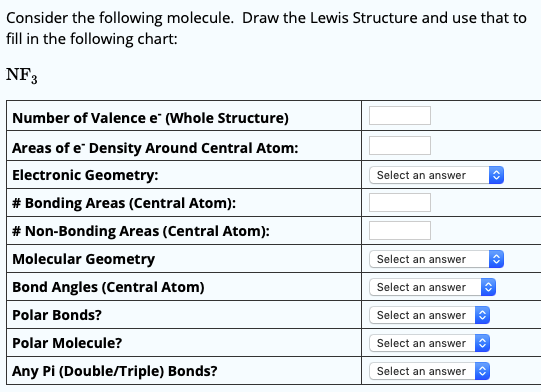
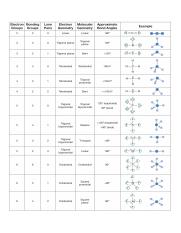
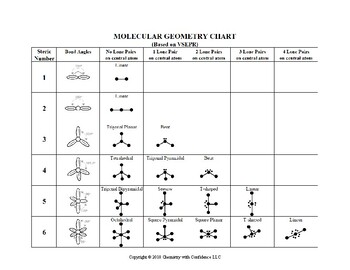
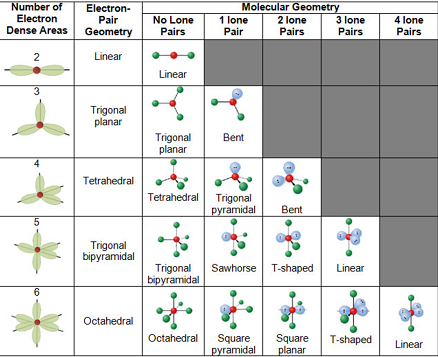
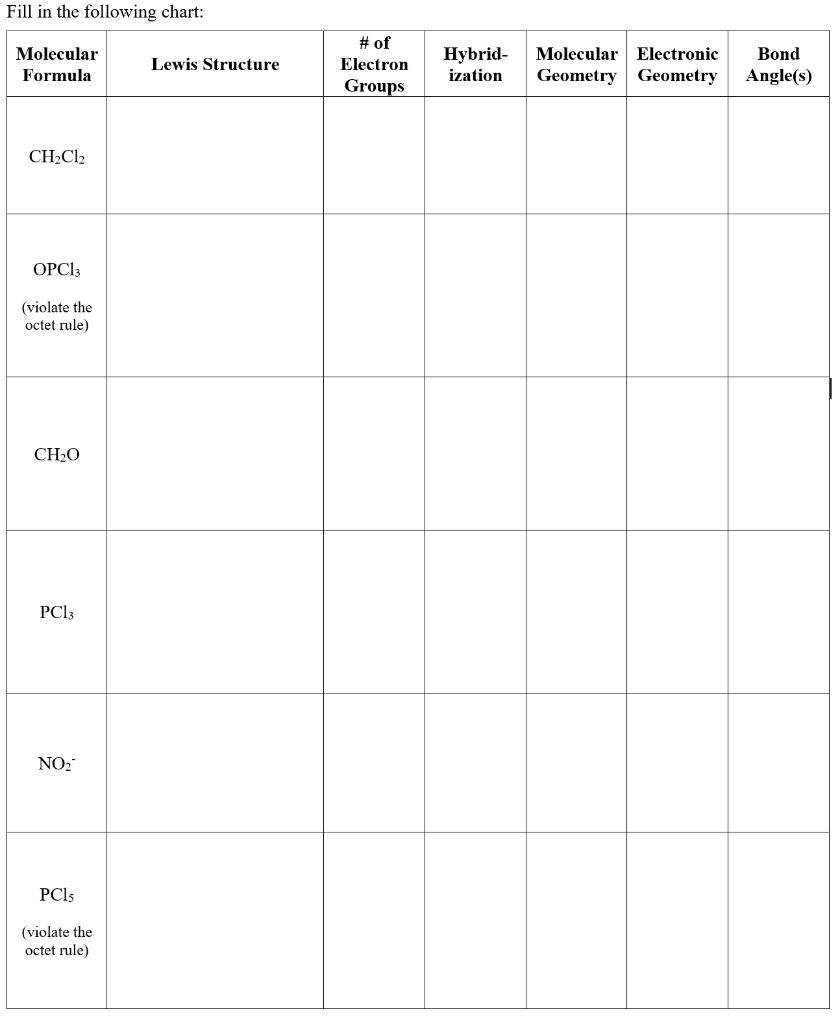
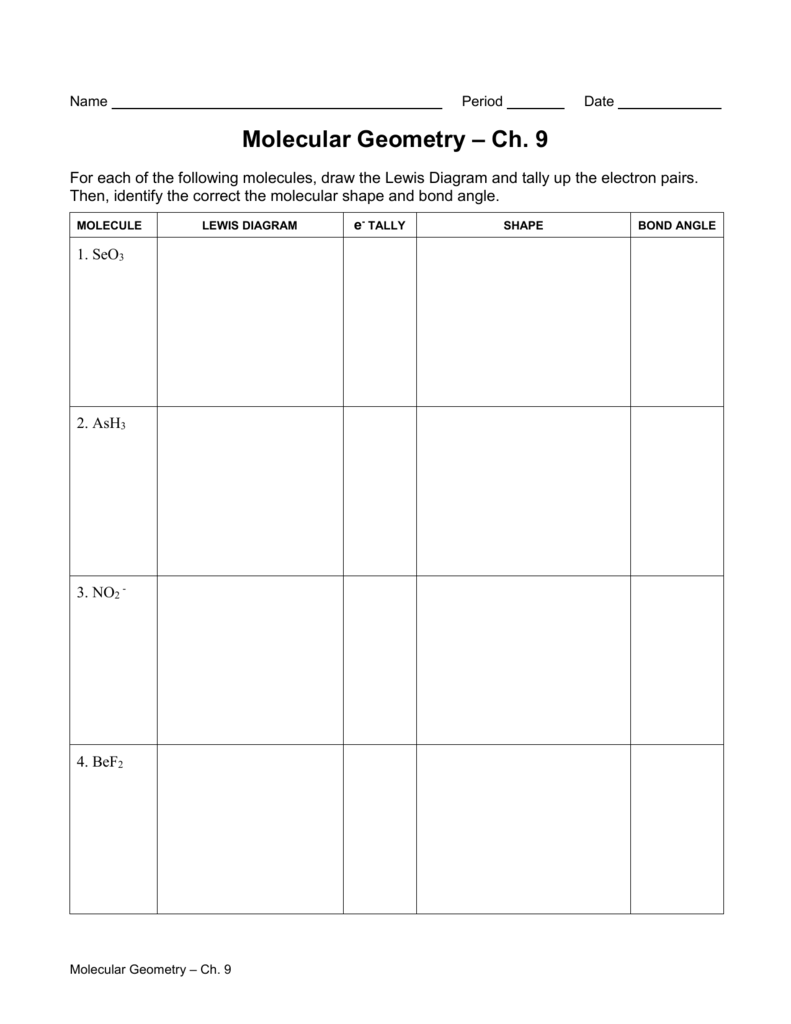
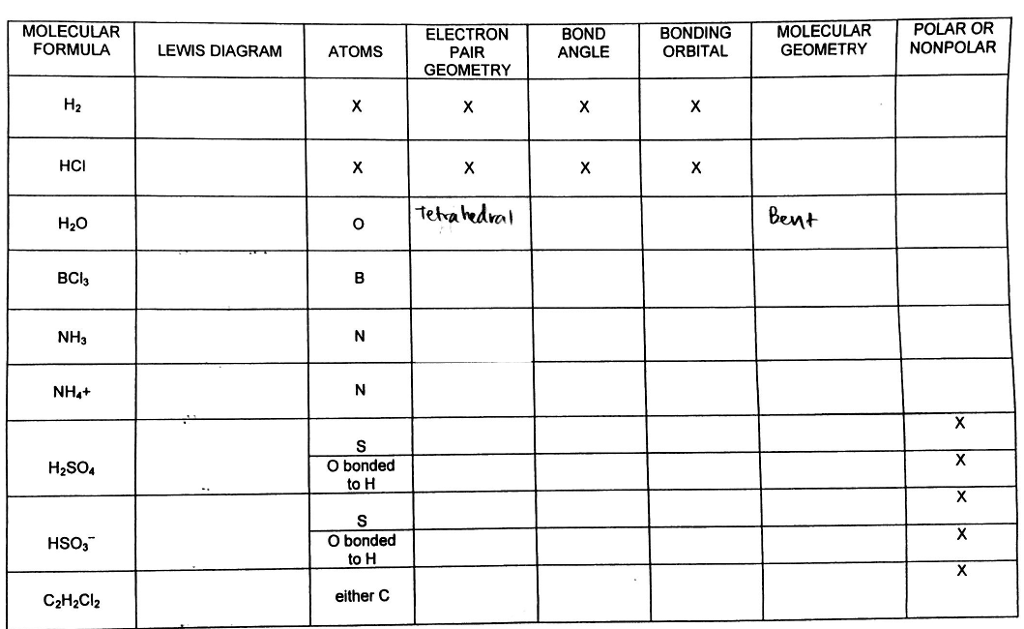
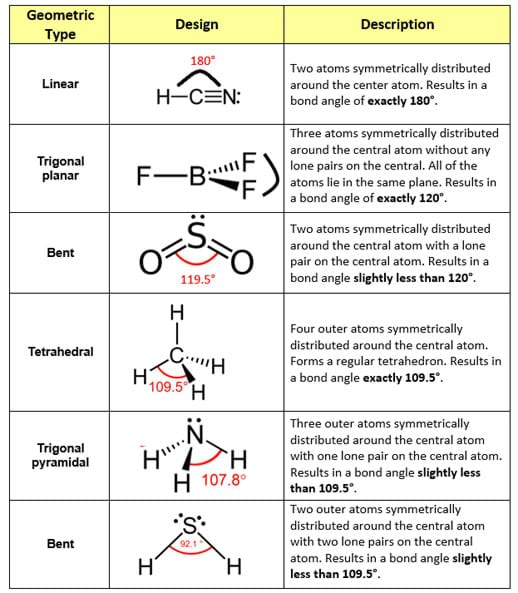
Bond Angles And Geometry Chart
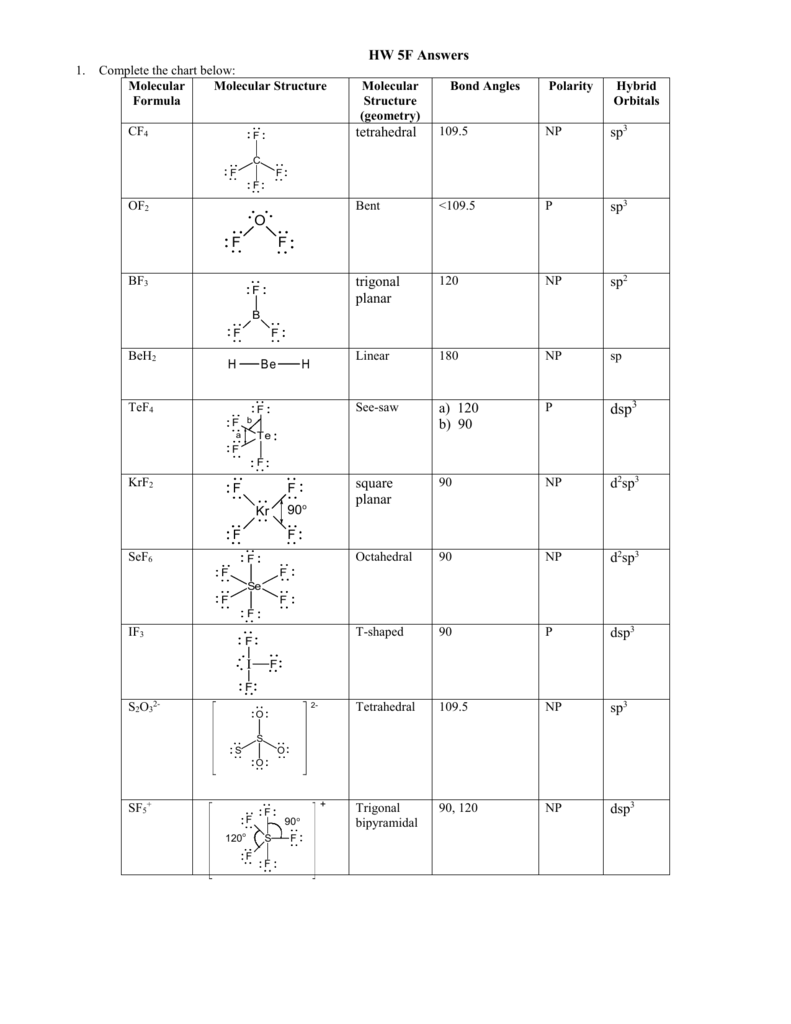
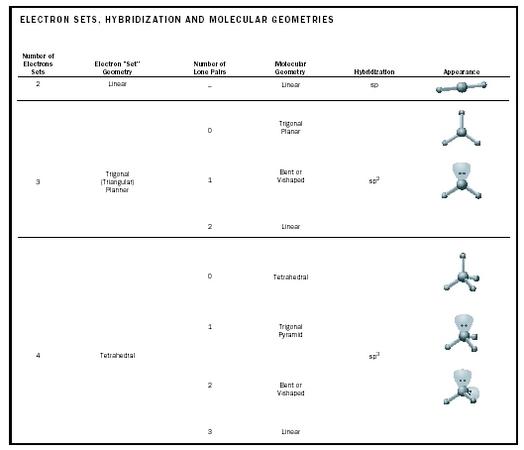
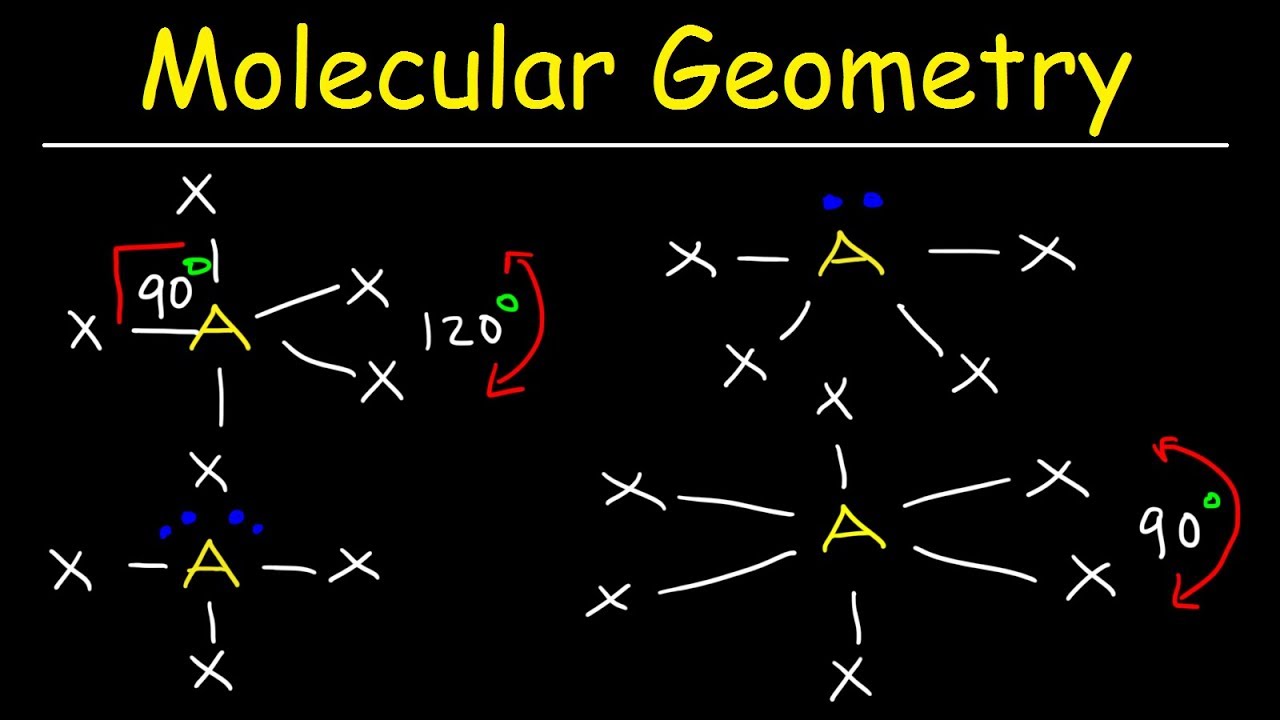
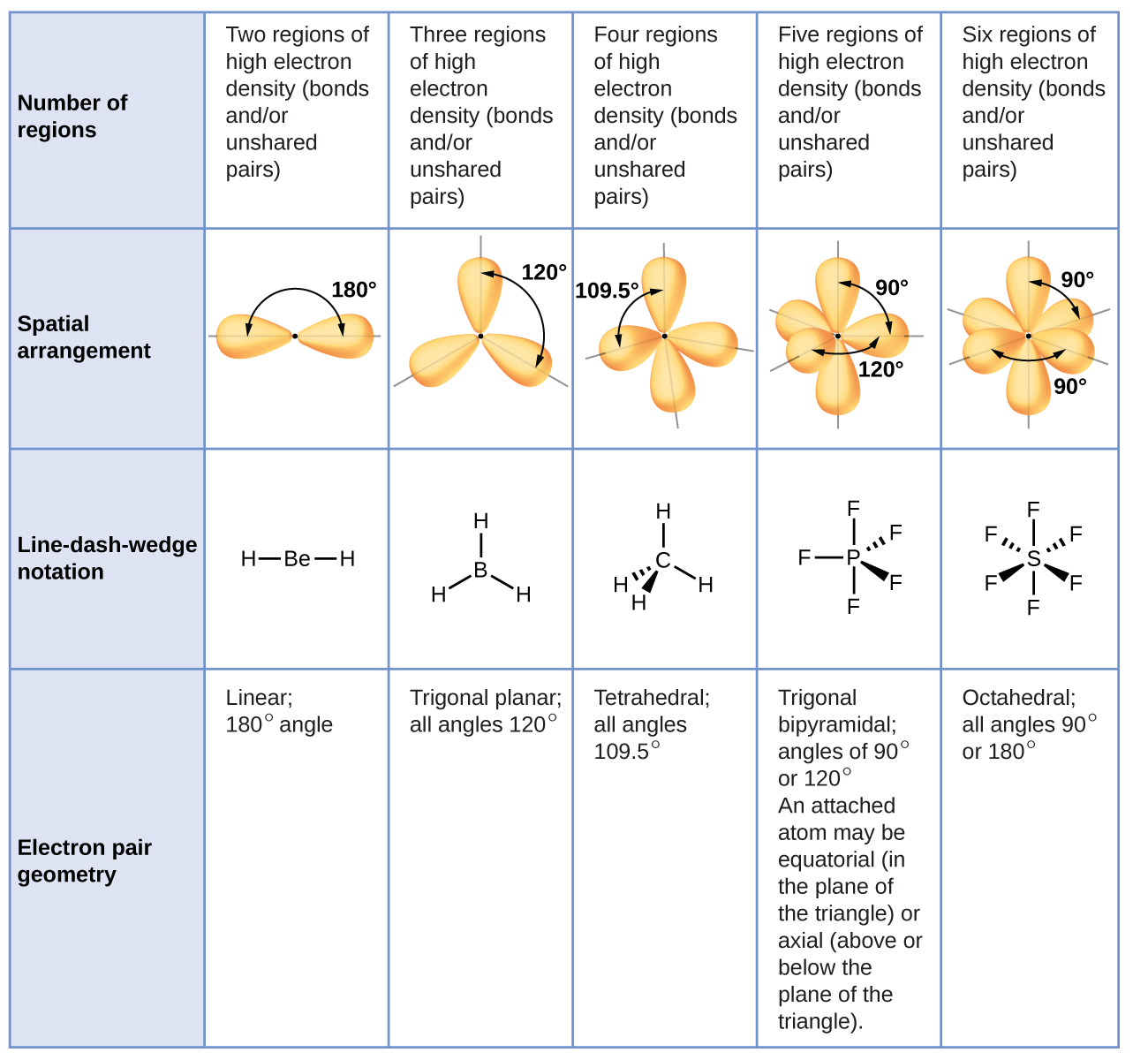
The bond angle can help differentiate between linear trigonal planar tetraheral trigonal bipyramidal and octahedral.
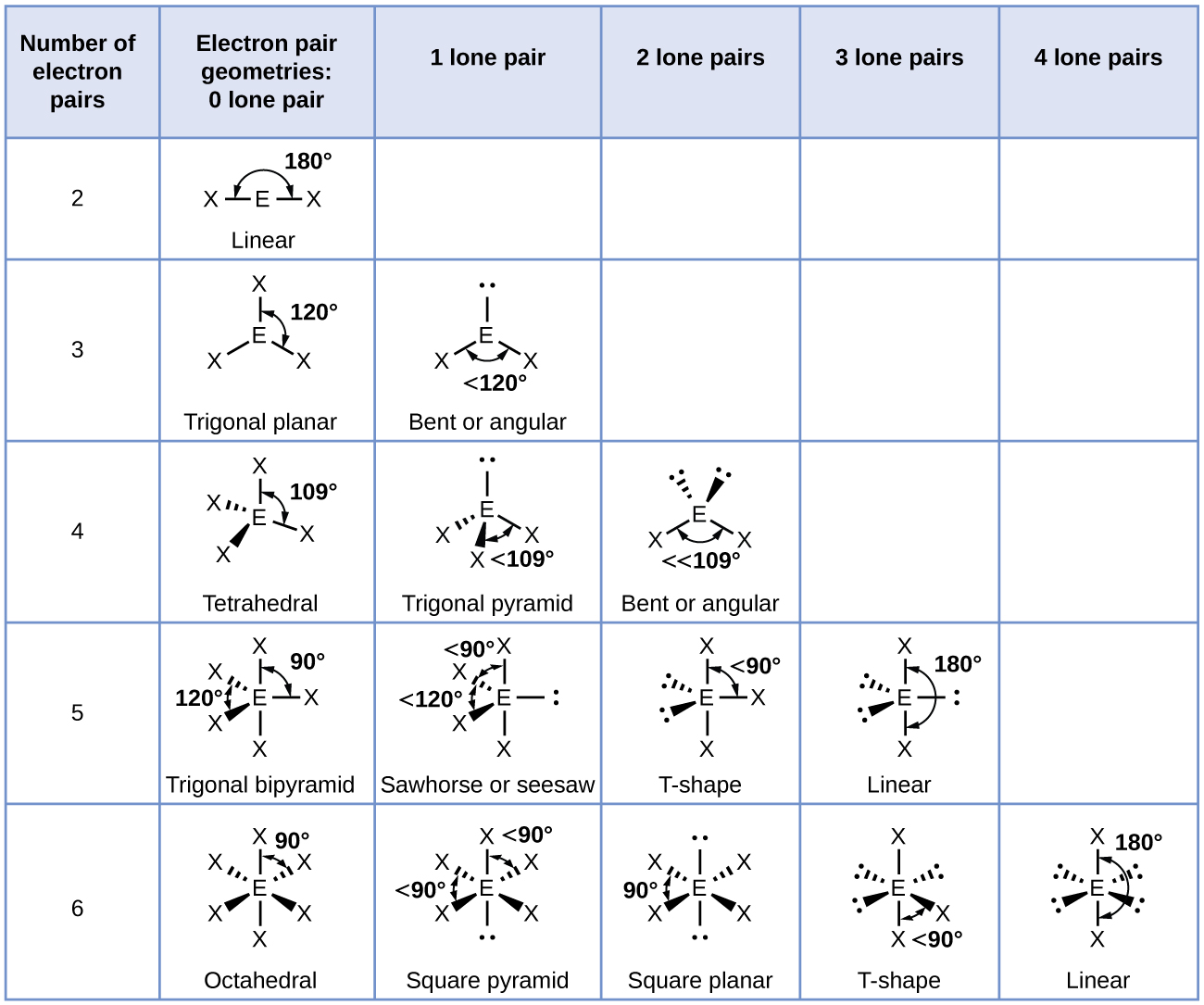
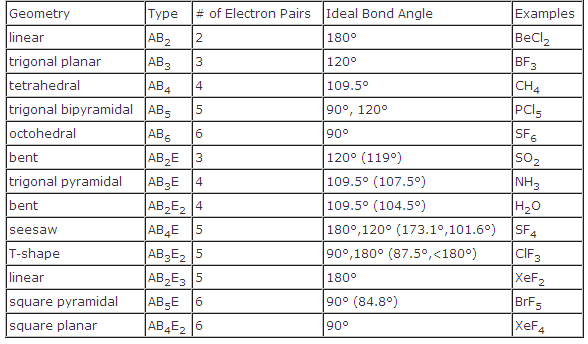
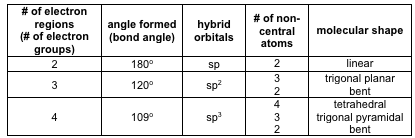
Bond angles and geometry chart. Its bond angles are 90 and 120 where the equatorial equatorial bonds are 120 apart from one another and all other angles are 90. Vsepr the shape of a molecule may be predicted by the number of atoms and un bonded electrons that surround an atom. More vsepr examples some other examples shown on the vsepr chart are sulfur hexafluoride sf 6 whose six electron pairs give it octahedral geometry with 90 angles and co 2 which has two electron pairs.
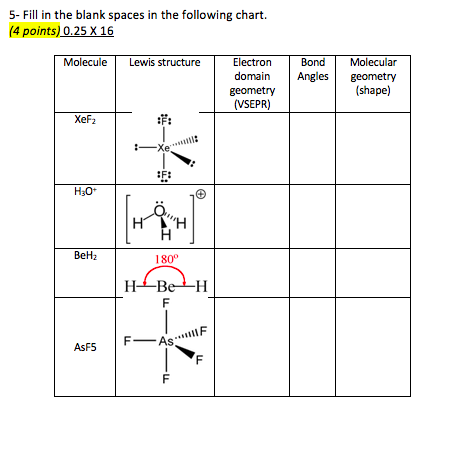
Hybridisation geometry shape structure and bond angle of molecules with example chemical bonding vsepr theory part 4 for neet iit jee iit jam ups. Xef4 molecular geometry bond angles section 2. Working with three dimensional models is a great way to get used to thinking about geometry in a three dimensional plane.
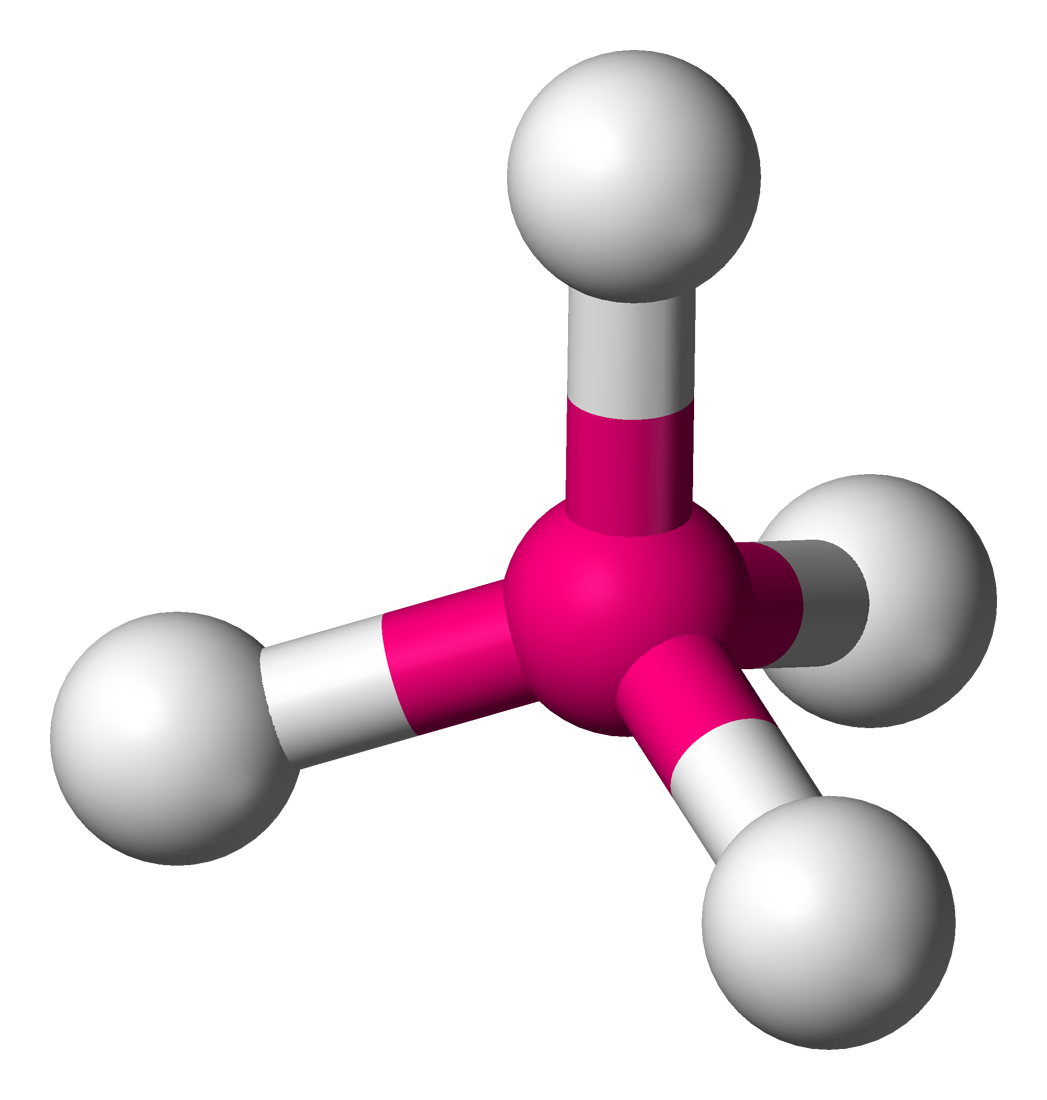
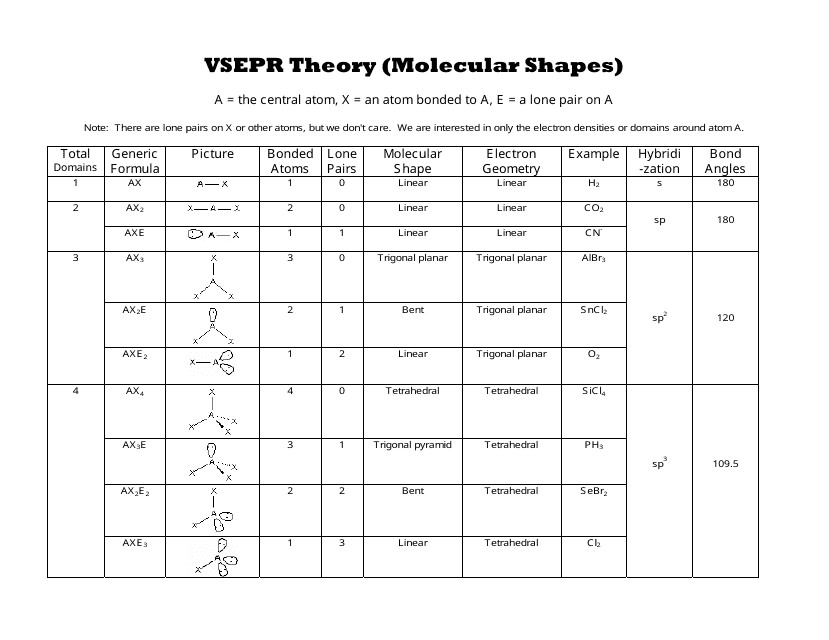
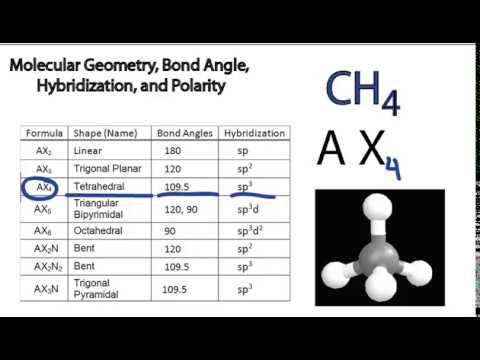
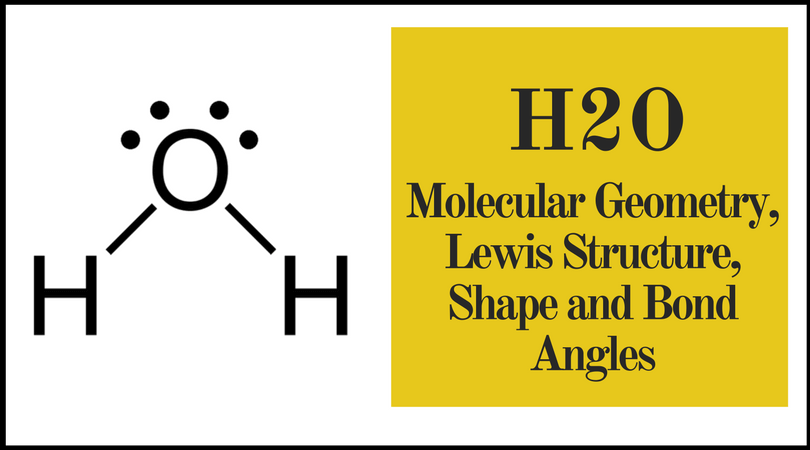
Vsepr theory molecular structure and polarity note. Each carbon is the center of a linear geometry. For example methane ch 4 is a tetrahedral molecule.
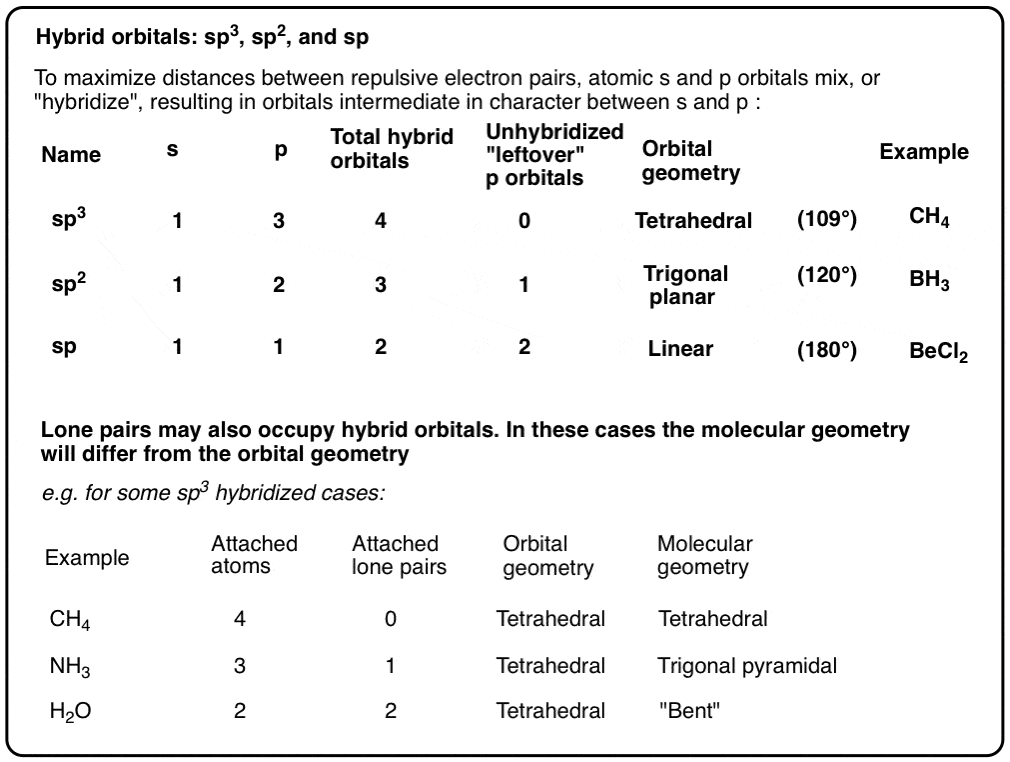
For trigonal pyramidal geometry the bond angle is slightly less than 109 5 degrees around 107 degrees. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion thus verifying the vsepr theory. In accordance with the vsepr valence shell electron pair repulsion theory the bond angles between the electron bonds are arccos 1 3 109 47.
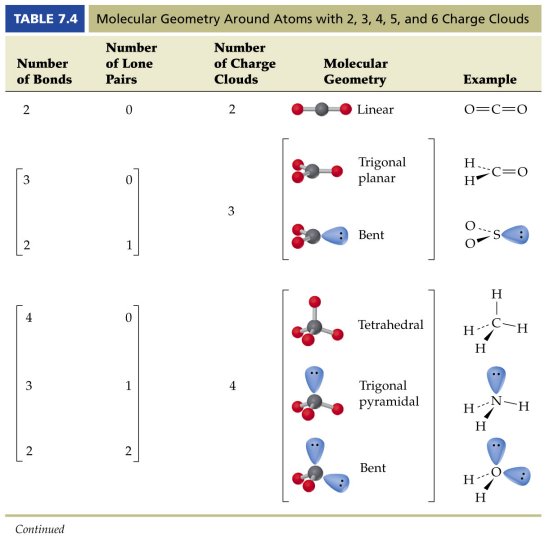
The other two atoms are on opposite ends of the molecule. Geometry example hybridi zation bond angles 1 ax 1 0 linear linear h 2 s 180 ax 2 2 2 0 linear linear co 2 axe 1 1 linear linear cn sp 180 ax 3 3 0 trigonal planar trigonal planar albr 3 ax 2e 2 1 bent trigonal planar sncl 2 3 axe 2 1 2 linear trigonal planar o 2 sp2 120 ax 4 4 0 tetrahedral tetrahedral sicl 4 ax 3e. This portion of the molecule is flat with bond angles of 120 degrees.
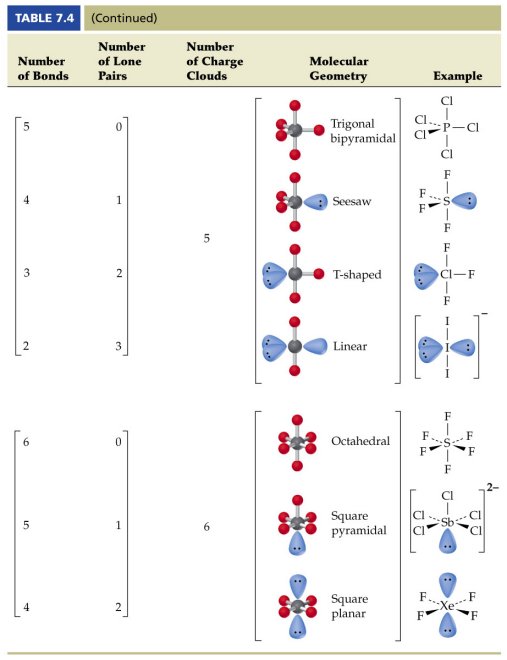
A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. We can draw the lewis structure on a sheet of paper. Examples include pcl 5 and asf 5.
Alkyne ch cch 3. Lets consider the lewis structure for ccl 4. For bent molecular geometry when the electron pair geometry is tetrahedral the bond angle is around 105 degrees.
Each student is to receive a molecular geometry handout valence shell electron pair repulsion theory. The bond angle is 90 degrees. The main centers of interest are the carbons of the triple bond.
Some elements in group 15 of the periodic table form compounds of the type ax 5. There are also apps and software you can use to create virtual models and get used to these concepts. A molecular geometry chart with bond angles will help to clarify these structures.
This portion of the molecule is in a straight line with bond angles of 180 degrees.




























/close-up-of-black-atom-model-on-table-680830745-58444c1d5f9b5851e542b740.jpg)